Outline of Research
Metastasis is the stage of cancer that kills most patients. However, this process is a bottleneck that only cells with specific properties can overcome. The role of cancer stem cells and non-cancer stem cells in this process is still under debate. Our group analyses the molecular mechanisms involved in tumor progression and metastasis and the impact of cancer cell plasticity. In particular, we focus on cell surface receptors such as adhesion molecules (CAMs) that mediate cell-cell and cell-extracellular matrix contacts. As they are tightly linked to signaling pathways, they can induce signaling in response to the microenvironment. One such CAM is CD44, a family of transmembrane glycoproteins that mediate proliferation, differentiation, migration and survival. Members of the CD44 family differ in the extracellular domain, where ten variant exons undergo alternative splicing.
We have discovered that several CD44 isoforms act as co-regulators of several signaling pathways, including receptor tyrosine kinases such as MET, VEGFR-2, EGFR, G protein-coupled receptors such as CXCR4 and members of the Wnt signalling pathway such as LRP6. CD44 isoforms act as signaling hubs that integrate signals from the microenvironment.
We aim to elucidate the molecular mechanisms of action of CD44 isoforms and to design inhibitory molecules that could be used in diseases such as cancer (pancreatic, colorectal, breast and leukemia) and liver disease.
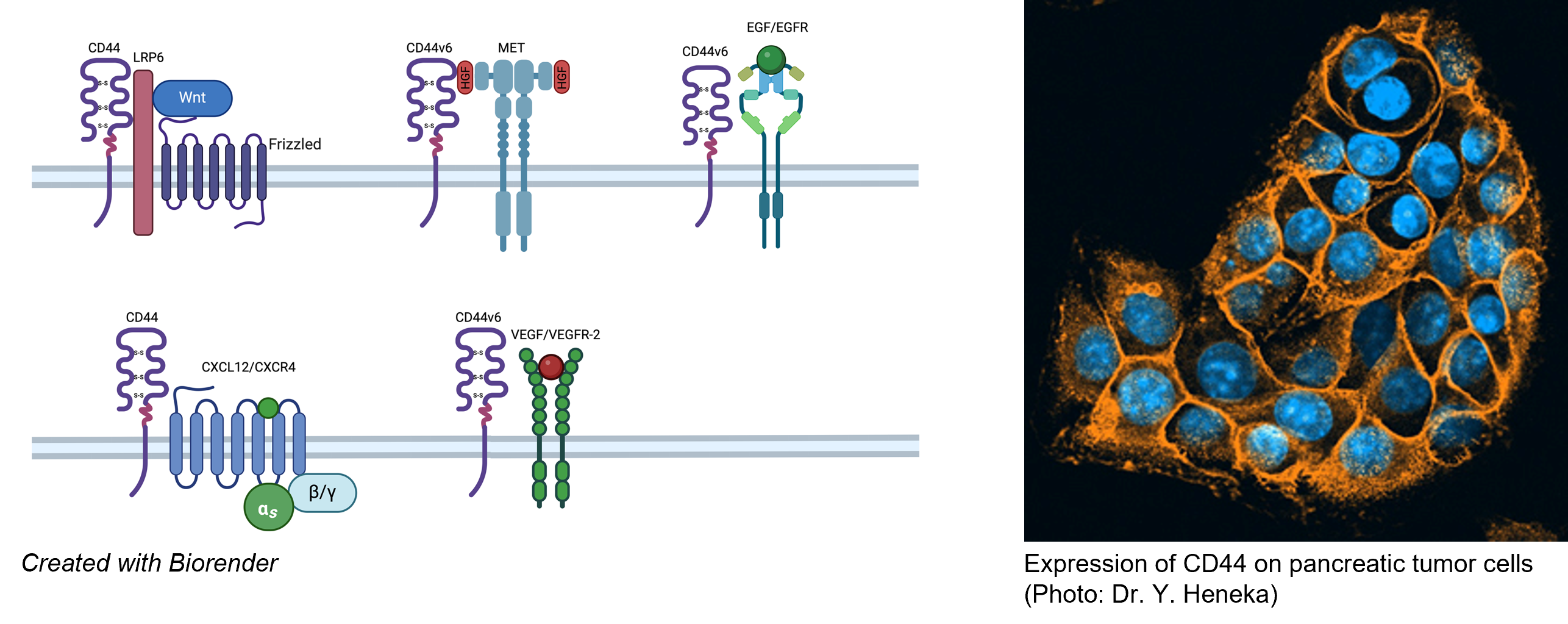
Figure 1: Molecular mechanism of action of CD44 isoforms:
CD44 and Receptor tyrosine kinases: CD44v6 acts as a co-receptor for several receptor tyrosine kinases (RTKs), including VEGFR2 and Met, two important RTKs that are critical for angiogenesis and metastasis. The role of CD44v6 for these RTKs is twofold: The ectodomain of CD44v6 is involved in RTK activation and the cytoplasmic domain promotes signaling after binding to the cytoskeleton via ERM (Ezrin-Radixin-Moesin) proteins. CD44v6 is involved in tumor progression and metastasis through its interaction with RTKs. We have developed a CD44v6-derived peptide that inhibits tumor growth, blocks metastasis and even eliminates established pancreatic cancer metastases. A modified version of this CD44v6 peptide is in phase I/Ib clinical trials (https://clinicaltrials.gov/ct2/show/NCT03009214?term=amcure&rank=1). CD44 G-protein-coupled receptors: Hyaluronan (HA), the major ligand for CD44, enhances CXCL12-induced CXCR4 signaling, a step required during angiogenesis. In contrast, small HA fragments (sHA) have the opposite effect. CD44-Wnt signaling: CD44 is involved in the regulation of LRP6 maturation and activation.
The themes developed in the group are:
1) Function of CD44 in plasticity of colorectal cancer
By means of engineered colorectal cancer organoids, we address the role of CD44 in colorectal cancer. We focus on the function of CD44 in the de-differentiation of non-cancer stem cells into cancer stem cells and the impact of this process on tumor growth and metastasis.

Figure 2: Colorectal cancer organoids (Photo Dr. S. Sonnentag)
2) Contribution of CD44 to the tumor microenvironment and to the metastatic niche
CD44, which is expressed on cancer cells, is highly involved in tumor initiation, progression and metastasis. However, its function is not limited to cancer cells. Activated stromal cells and infiltrating immune cells form a tumor microenvironment on which cancer cells depend. In pancreatic ductal adenocarcinoma (PDAC), the most deadly form of cancer, most of the tumor volume is made up of tumor stroma, which is composed of cancer-associated fibroblasts (CAF), pancreatic stellate cells (PSC; Figure 4), tumor-associated macrophages (TAM) and other immune cells. The difficulty in treating PDAC stems from its early spread to the liver, where a pre-metastatic niche is formed by haematopoietic cells derived from the bone marrow. All of these cell types express multiple CD44 isoforms, which may be involved in the regulation of several molecular pathways that are key to their tumor-promoting function. Given that CD44 blockade has been shown to be highly effective in inhibiting tumor progression and metastasis, we are investigating the role of stably expressed CD44 in stromal cells during tumor development and metastatic niche formation.
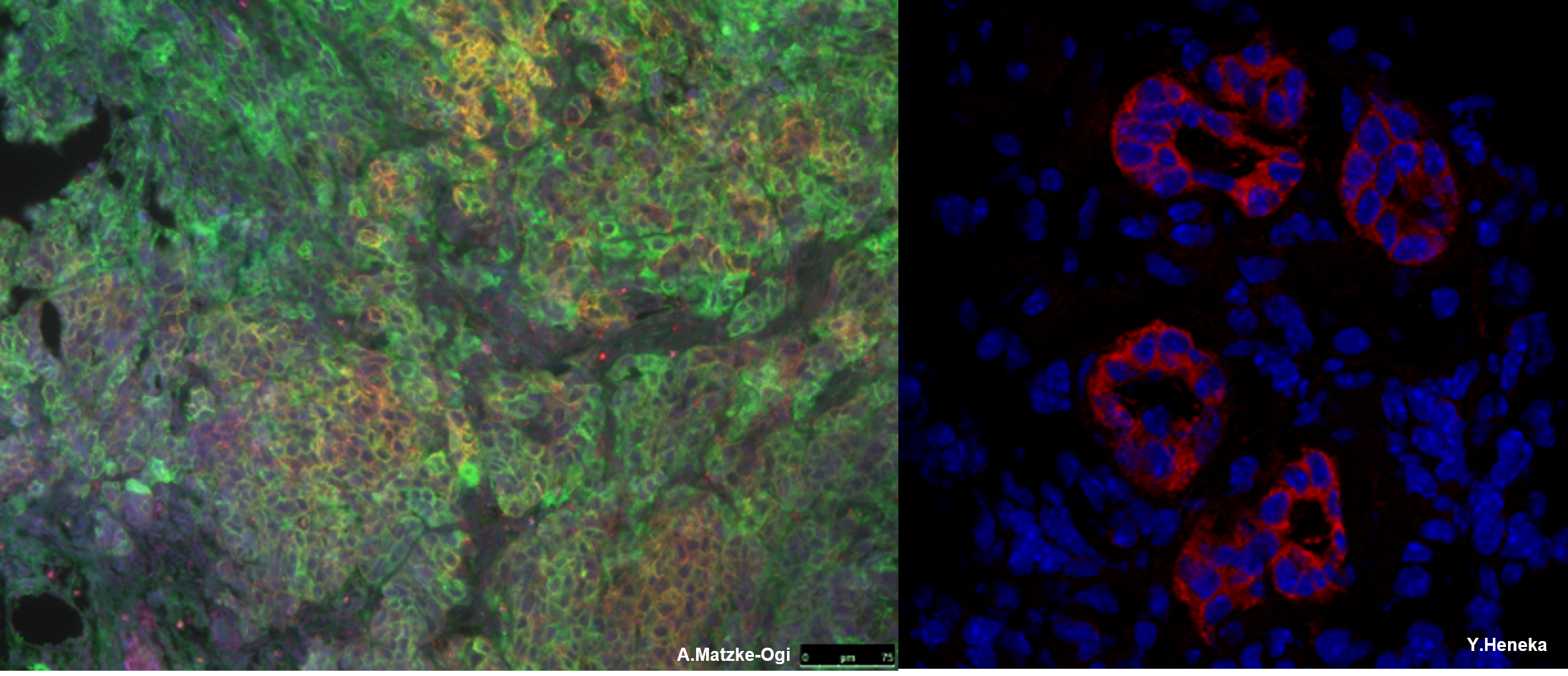
Figure 3: Pancreatic tumor expressing CD44v6 and MET (left), CD44 expression in blood vessels from a pancreatic tumor (right)
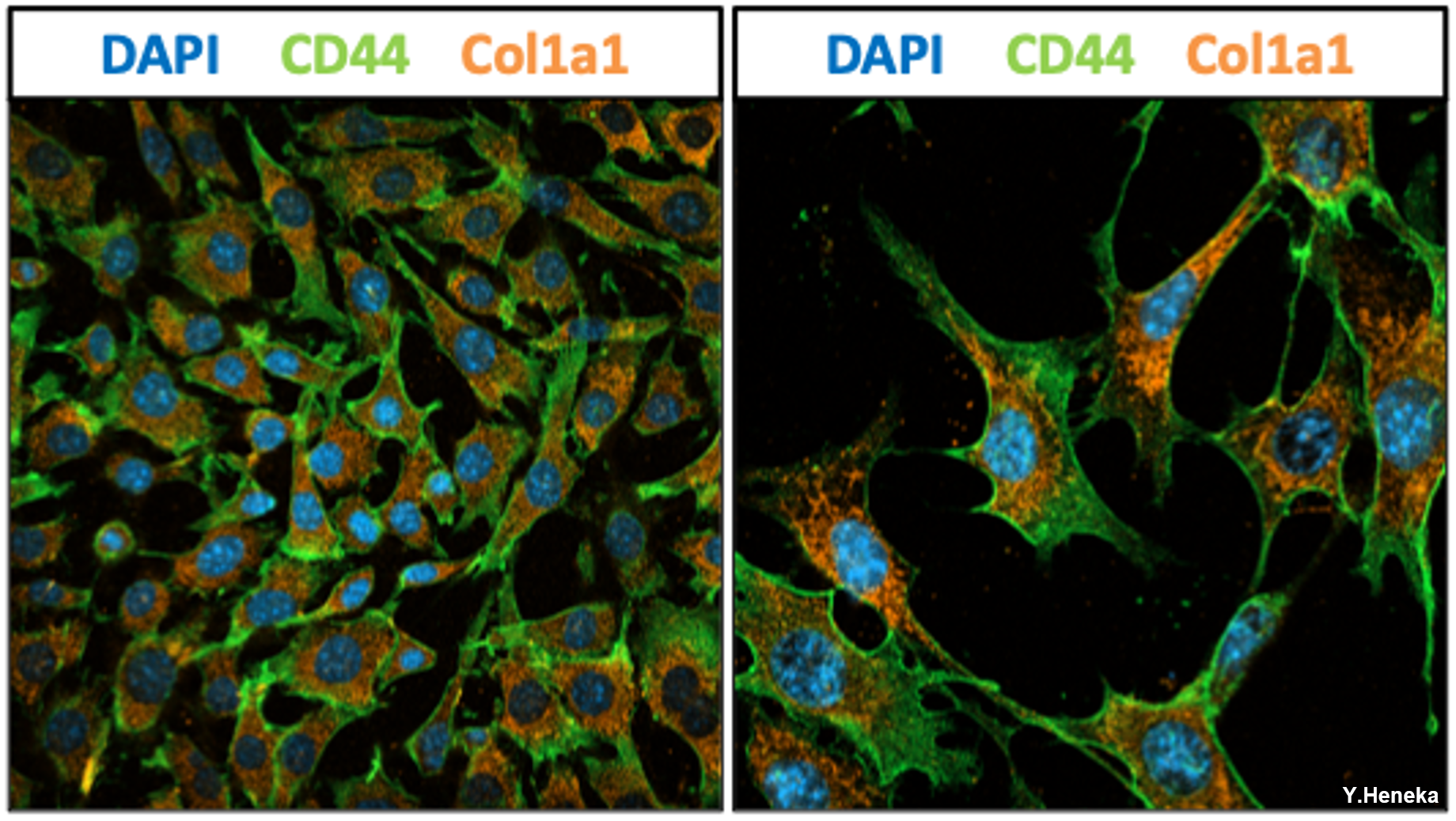
Figure 4: Pancreatic Stellate Cells expressing CD44 and the activation marker Col1a1.
3) CD44 and HA in chemokine signaling
The interplay between HA, CD44, CXCL12 and CD44 has consequences on the homing of hematopoietic stem cells to their niche and in hematological diseases (chronic lymphocytic leukemia, acute myeloid leukemia). The collaboration between these molecules plays a role in the resistance of leukemic stem cells to chemotherapy.
4) CD44 in Wnt signaling
The molecular mechanism of action of CD44 in Wnt signaling is studied during the development and homeostasis of the intestine and in intestinal stem cell function.
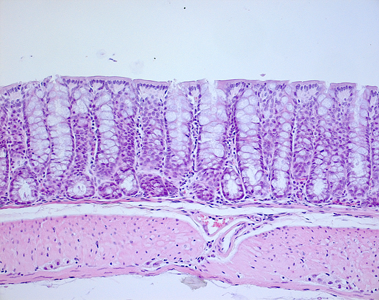
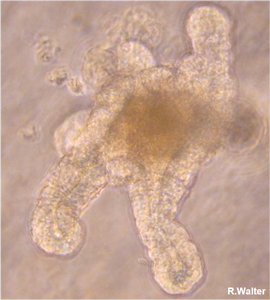
Figure 5. section of a colon and organoid
Given the importance of CD44 in intestinal stemness, the consequences of a removal of CD44 in the intestinal epithelium is investigated in intestinal regeneration.
In addition, the crosstalk between CD44 and Wnt is studied in inflammatory bowel diseases and Wnt-driven colorectal cancer. There, special attention is focused on cancer cell plasticity.
Tools:
We have developed a panel of engineered cancer organoids. We have established a Cd44 floxed and a Cd44v6 floxed mouse and study the role of CD44 in development upon conditional activation of Cd44 in liver, intestine, breast and skin. We also have developed a panel of CRISPR/Cas constructs against various CD44 isoforms.